16 Which of the Following Equals One Atomic Mass Unit
What unit is used to measure weighted average atomic mass. It replaces the atomic mass unit without the unified part and is.

One Atomic Mass Unit Is Equal To Given Mass Of One Carbon 12 Atom 1 992 10 23
6 6 1 0 2 4 g.

. Mitgliedd1 and 8 more users found this answer helpful. The carbon-12 atom has six neutrons and six protons in its nucleus. Atomic Mass Unit u Gram g 001 u.
One atomic mass unit or amu is equal to one-twelfth of the mass of Carbon - 12 isotope. Find the atomic mass of each element in the molecule. An atomic unit of mass is defined as accurately 112 the mass of a carbon-12 atom.
Which of the following equal one atomic mass unit. The mass of one helium-4 atom. 1 amu Average of the proton rest mass and the neutron rest mass.
However Wiki shows that which already differs from the second decimal. C one-sixth the mass of one helium-4 atom. Mass and relative abundance of each isotope of that element.
Find the mass combination of each element. One atomic mass unit is equal to 1. An atomic mass unit equals 1661 1024 g.
Solution for If an atomic mass unit equals 166 1024 g what is the mass in grams of 500 1022 formula units of NaOH. A one-twelfth the mass of one carbon-12 atom B the mass of one electron C akashskyash534 akashskyash534. For example one atom of helium-4 has a mass of 40026 amu.
So atomic mass unit 112 12gmol 1gmol. The mass of any isotope of any element is expressed in relation to the carbon-12 standard. An atom of sulfur-32 has a mass of 31972 amu.
One atomic mass unit is equal to a twelfth of the mass of a carbon 12 atom. 120107 Atomic Mass of H. 1 amu 167377 x 10 -27 kilogram or 167377 x 10 -24 gram.
One-twelfth the mass of one carbon-12 atom Electrons are negatively charged and have a mass of 1 amu. We know mass of carbon -12 isotope 12gmol. 1 amu16726219xx10-27 kg.
2 Mass number equals the number of neutrons. Find an answer to your question Which of the following equals one atomic mass unit. An atomic mass unit equals 1661 1024 g.
1 atomic mass unit amu is the mass of a proton or a neutron which is equal to 16726219xx10-27 kg. One convenient aspect of atomic mass units is that while based on carbon mass a single unit is also equal to one hydrogen atom. Unified atomic mass unit u Dalton Da universal mass unit either amu or AMU is an acceptable acronym for atomic mass unit.
The mass of one electron. A one-twelfth the mass of one carbon-12 atom. What is the mass in grams of each molecule of a H2S b N2O4 c ICl3 d NCl3.
But also we can say that the molar mass of a hydrogen is equal to 1gmol and this is because. It is equal to 112th of the mass of an atom of carbon-12. Hence one atomic mass unit is numerically equivalent to 1gmol.
What is the mass in grams of a. It is actually the definition of an atomic mass unit. Atomic Mass of C.
Which of the following equals one atomic mass unit. The atomic mass of an element depends upon the _____. 100794 Atomic Mass of O.
16726219xx10-24 gxx6022xx10231007gmol which is close to the mass. In the Bohr model of the atom an electron in a orbit has a fixed. Filling these weights in the above formula shows that.
B the mass of one electron. Atomic mass unit is recognized now as a non-SI unit. Which of the following equals one atomic mass unit.
Actually atomic mass unit or amu is the mass of one nucleon either a single. This is because the combined mass of a single proton and neutron the composition of a hydrogen atom is equal to the measurementElectrons being only 11836 the mass of a proton are essentially negligible to the overall mass of an atom. It was once based on the hydrogen atom but today scientists define it using the carbon 12 atom.
D the mass of one carbon-12 atom. The mass of one carbon-12 atom d. The atomic unit mass is symbolized as amu.
Hence the above statement is. The mass of a Hydrogen atom is 1 amu. It is abbreviated as amu.
The mass of one helium-4 atom c. An atomic weight unit is the. The proton and the neutron each have a mass approximately equal to one Atomic Mass unit.
One-twelfth the mass of one carbon-12 atom. Chemistry questions and answers. 3 An atomic mass unit equals 112 the mass of a carbon-12 atom a 1 only b 2 only c 3 only d 1 and 2 e 2 and 3 26.
Atomic Mass Unit u Kilogram kg 001 u. One atomic mass unit is a mass unit which is equal to the mass of exactly one-twelfth 112th of the mass of one atom of carbon-12 isotope. It is equal to 112th of the mass of an atom of carbon-12.
The unified atomic mass unit is a physical constant that is accepted for use in the SI measurement system. Which of the following equals one atomic mass unit. Calculate the relative molecular mass of a gas if a 500cm3 sample at 20ªC and 1 atomic mass of 066g.
A atom consists of 6 protons 6 neutrons and 6 electrons. 1 u 1 Da 1 amu in modern usage 1 gmol. An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon-12.
The mass of one carbon-12. The atomic mass unit is equal to one-twelfth the mass of a carbon-12 atom or 1660538921731027 kg. The mass of one electron b.
1 Atomic number equals number of protons plus neutrons. One atomic mass unit is 112 of the weight of a atom in its ground state. I Atomic mass of H 1 g atomic mass of O 16 g atomic mass of Cu 635 g atomic mass of S 32 g now molar mass of CuSO45H2O atomic mass of Cu atom mass of S 4 atomic mass of O 5 2 atomic mass of H atomic mass of O 635 32 4 16 5 2 16 g 635 32 64 90 g 2495 g.
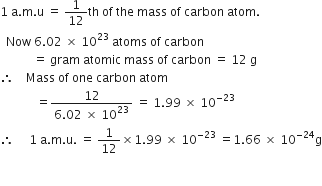
Calculate The Mass Of 1 A M U In Grams From Chemistry Some Basic Concepts Of Chemistry Class 11 Cbse

Calculate The Formula Unit Masses Of Zno Na2o K2co3 Given Atomic Masses Of Zn 65u Na 23 U K 39 U C 12 U And O 16 U

Atoms And Molecule Class Ix Mcq

Atomic Mass Unit Or Unified Mass Unit Chemistrygod

Show That Energy Equivalent Of One Atomic Mass Unit Is Nearly 933mev Youtube
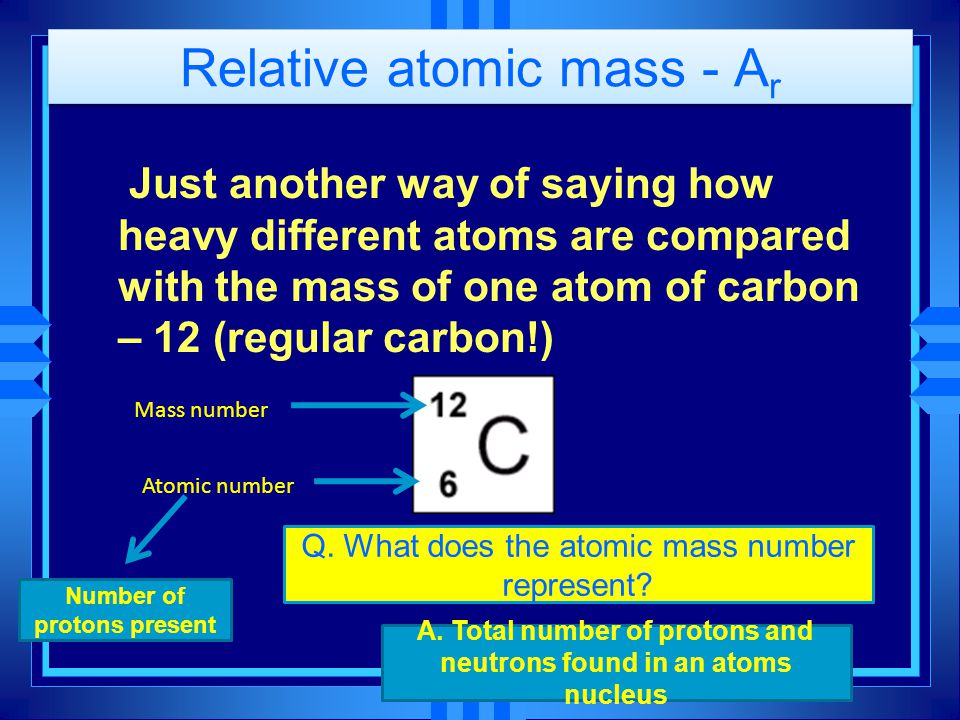
Relative Atomic Mass A R Just Another Way Of Saying How Heavy Different Atoms Are Compared With The Mass Of One Atom Of Carbon 12 Regular Carbon Ppt Download

Atomic Mass Unit Or Amu In Hindi Youtube

Calculate The Energy Equivalent Of 1 A M U In Mev Youtube

Find The Energy Equivalent Of One Atomic Mass Unit First In Joule And Then In Mev Using This Express The Mass Defect Of 8o 16 In Mev C 2 Given M P 1 007825u And M N 1 008665 U

Chemistry Review 1 The Atom Nuclear Electron Config 2 Matter Phases Types Changes 3 Bonding Periodic Table Ionic Covalent 4 Compounds Formulas Ppt Download

Chemistry Chapter 4 Barnstable Academy

Atomic Mass Standard Mass Unit Is Derived From Carbon 12 Atomic Mass Unit The Mass Equal To 1 12 The Mass Of One Carbon 12 Atom Ppt Download
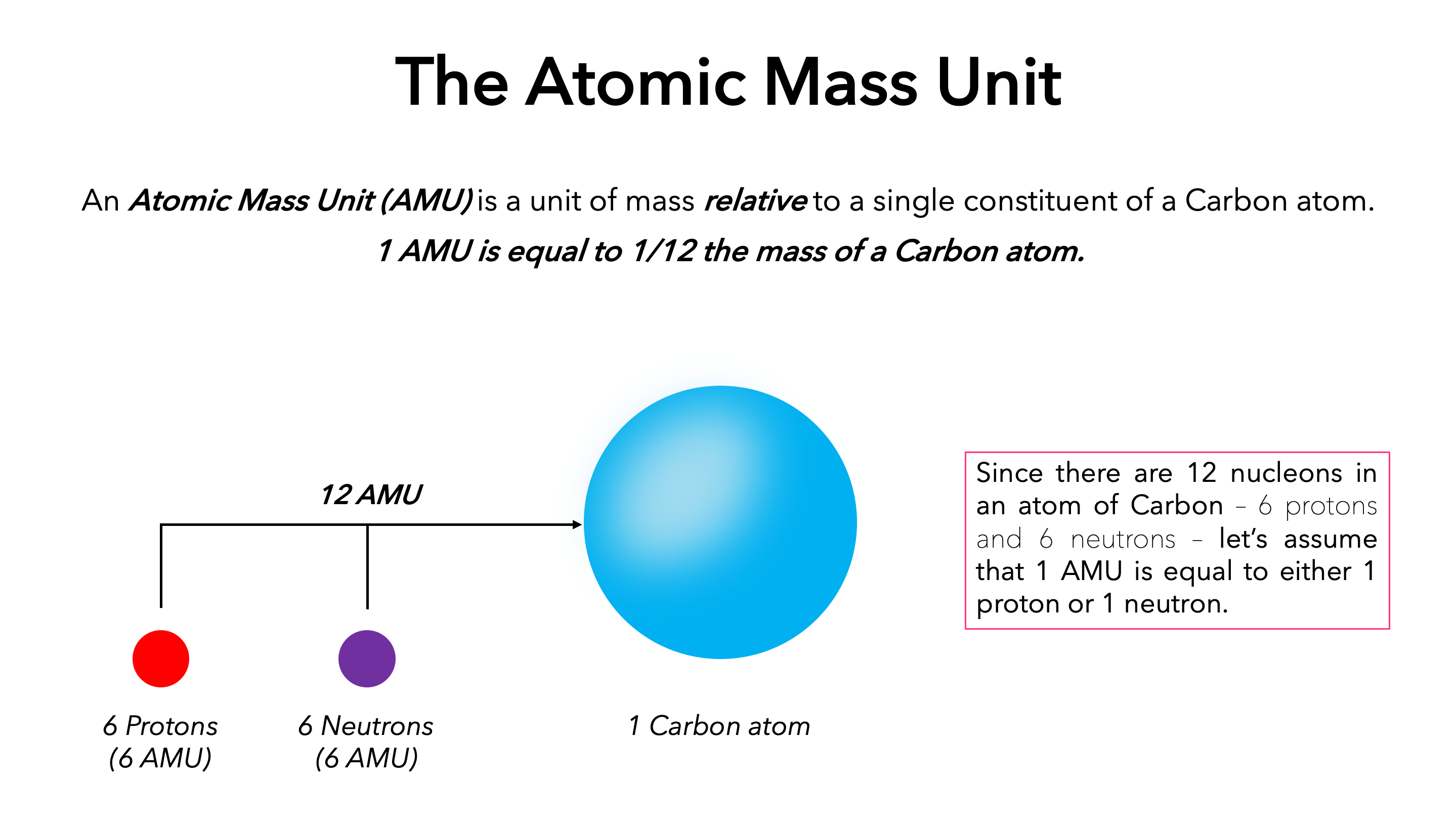
Atomic Mass Definition Amu Expii
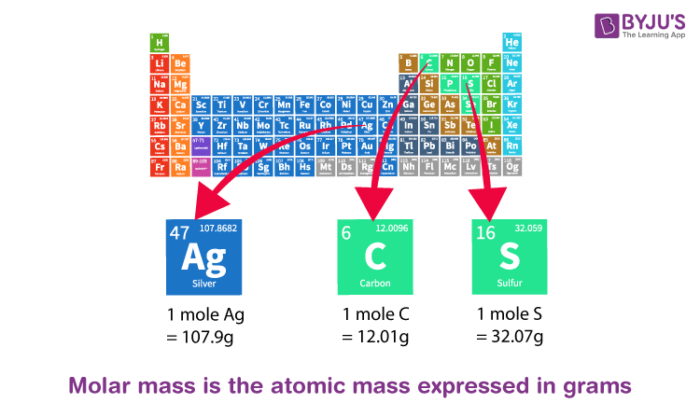
What Is Atomic Mass List Of Elements Sorted By Atomic Mass Of Iron Sulphur Potassium Chlorine Etc

Find The Energy Equivalent Of One Atomic Mass Unit First In Joule And Then In Mev Using This Express The Mass Defect Of 8o 16 In Mev C 2 Given M P 1 007825u And M N 1 008665 U

How Does 1 Amu 1 Avagadro S Number Quora

Atomic Mass Standard Mass Unit Is Derived From Carbon 12 Atomic Mass Unit The Mass Equal To 1 12 The Mass Of One Carbon 12 Atom Ppt Download
How Come The Mass Of A Single Atom Of An Element In Amu Is Numerically Equal To The Mass Of One Mole Of Those Atoms In Grams Quora

Comments
Post a Comment